BIA-ALCL: Breast Implant-Associated Anaplastic Large Cell Lymphoma
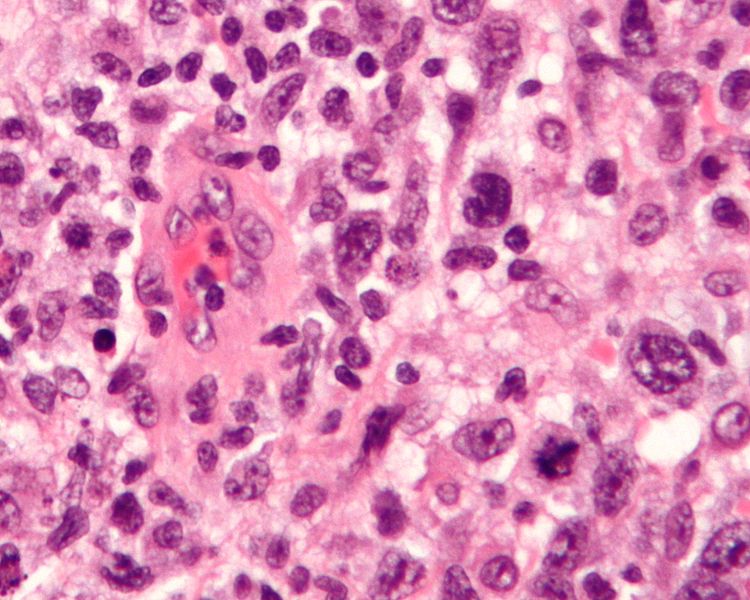 Breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) is a rare condition which can develop following the placement of textured breast implants. This type of anaplastic large cell lymphoma typically deveolps near the implant within the capsule that the body naturally forms around the implant. The lymphoma forms within scar tissue of the implant capsule rather than in the breast tissue itself. BIA-ALCL is not breast cancer.
Breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) is a rare condition which can develop following the placement of textured breast implants. This type of anaplastic large cell lymphoma typically deveolps near the implant within the capsule that the body naturally forms around the implant. The lymphoma forms within scar tissue of the implant capsule rather than in the breast tissue itself. BIA-ALCL is not breast cancer.
The FDA classifies BIA-ALCL as an uncommon cancer. The organization believes that women with textured breast implants have a small but increased risk of developing this disease in the tissue capsule the body forms around a textured implant over time. At this time, data indicates that the incidence of ALCL is low, even among breast implant patients. Between one in 355 to one in 2,200 of these individuals develop the disease depending on type of textured implant, according to recent studies. Currently, there does not seem to be a greater cancer risk based on the type of implant (silicone or saline) or the type of surgery (breast augmentation or reconstruction). According to the U.S. Food and Drug Administration (FDA), as of September 2020 more than 700 people worldwide have been diagnosed with breast implant-associated anaplastic large cell lymphoma (BIA-ALCL).
Persistent pain or swelling around a breast implant, on one side only, is the most common symptom of BIA-ALCL. This would occur not less than one year after the textured implant placement, but typically around 8-9 years after placement of a textured implant. Patients also sometimes report a lump under the skin or capsular contracture usually on one side only. So far, the FDA has found no significant link between silicone implants or saline implants and the development of this condition, only the type of outer covering of the implant (textured vs. smooth). If you are unsure what type of implants you have, contact the plastic surgeon that placed them.
Since 2019, FDA has had textured breast implants removed from the USA market. All smooth breast implants are still considered safe by the FDA. At this time, the FDA is not recommending that women with any type textured implants, have them removed or exchanged, unless they are having symptoms or problems with the textured breast implants.
For more information on the FDA findings on implants, visit their website. You may also contact our office to speak with a member of our team if you have specific questions regarding your implants.